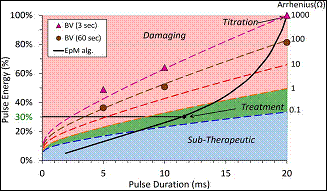
Fig. 1:
Legend
for Fig. 1:
Dashed lines,
corresponding to different clinical grades, differ by an order of magnitude in
Arrhenius integral X. The red
area corresponds to the damaging
settings and green to the nondamaging range of HSP expression; blue is
below the threshold for cell response. Titration of 100% corresponds to barely
visible lesion (BV) observed at 3 seconds.
Lavinsky D, Wang J, Huie P, et al. Nondamaging retinal laser
therapy:rationale and applications to the macula. Invest Ophthalmol Vis Sci.2016;57:2488–2500.
(Content is licensed under a Creative Commons Attribution 4.0
International licence. https://creativecommons.org/licenses/by/4.0/)
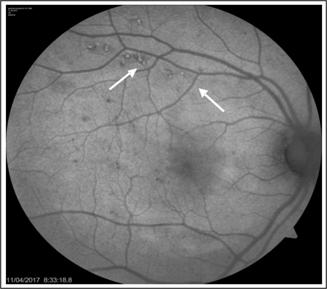
Fig. 2:
Legend for Figure 2:
Fundus autofluorescence image of a patient treated with NRT. White
arrows demonstrate hyperfluorescent test spots. No treatment effect is seen at
the macula.
Non-damaging retinal laser therapy (NRT) (previously
named EpM) (Manufacturing Company: Topcon)
Heating of biomolecules by laser energy leads to protein denaturation as a temperature dependent chemical reaction. Above a certain threshold cellular necrosis and coagulation
occurs. The
technique NRT is based on the Arrhenius equation which is a computational tissue temperature model that was obtained by animal experiments. By the Arrhenius
equation it has been shown that at a certain pulse duration, 30% of the barely
visible treatment effect which is termed as the threshold energy has been shown to be the highest non damaging and
also the level that has therapeutic effect illustrated by the green shaded area (Fig. 1). Above 30%
of the threshold energy has been shown to be damaging and below 30% of the
threshold energy has been shown to be sub-therapeutic24. By animal experiments of retinal laser therapy and
immuno-histochemical staining for heat shock proteins (HSP), it has been shown
that with 100% threshold energy, no HSP expression is seen over the laser
treated area demonstrating cell death over the laser treated area with HSP
expression sorrounding the laser burn, implying there has been sublethal
thermal elevation sorrunding the laser burns. With 30% threshold energy, as HSP
is expressed over the laser spots, it is shown that cells are not damaged over
the laser treated areas. Laser induction of HSP by thermal stress, is thought to rejuvenate RPE cells and restore their function25,26. Promising results have been reported for chronic
central serous chorioretinopathy and MacTel type 2 but there has been limited
experience24. NRT is applied in a grid pattern with 0.25 spot
spacing between spots, spot size is
200µ, duration is 15ms. Distance from foveal center is 500 – 700 microns. The
energy needed for a barely visible treatment effect, which is the threshold
energy is determined and 30% of the energy needed for a threshold burn is used
for treatment.
Retina rejuvenation therapy (2RT)
(Manufacturing Company:Ellex)
With 2RT, laser mode is discontinuous
and the pulse duration is even shorter, it has been reduced to 3ns. Subthreshold
laser power is used. This results in damage from cavitation rather than thermal interaction, only few RPE cells are damaged without causing Bruch’s membrane
rupture. Each of the dead RPE cell is surrounded by unaffected RPE cells. The overlying photoreceptors do not
undergo secondary cell death. A
pilot clinical study about the technique has been published, reporting visual
acuity improvement and reduction in macular thickness27.
Selective Retinal Therapy (SRT)
(Manufacturing Companies:Medical Laser
Center, Lumenis)
SRT has been
developed to further improve selectivity by using a much shorter pulse
duration of 1.7 μs, and consequently a higher
irradiance. It has been demonstrated in animal studies that
selective treatment of the RPE is achieved using microsecond
pulse durations, and the follow-up showed that the RPE regenerates with
survival of the adjacent photoreceptors28. There are few clinical studies evaluating SRT in
diabetic macular edema. Stabilisation of visual acuity or improvement in over
80% of patients have been reported. This treatment although described as
subthreshold, has FFA findings indicating RPE damage29.
Limitations of Subthreshold Lasers
The primary limitation is the absence of a
visible end point during treatment and determination of threshold energy out of the macular
area which leads to concerns of under treatment. For micropulse lasers the lack of standardized treatment parameters are
other major limitations as laser settings can be
different depending on the study, with various duty cycles, spot sizes, and durations. Large scale randomised controlled trials
are required for comparison with conventional laser, anti-VEGF treatment and
combined anti-VEGF with subthreshold laser therapy to find out the actual role
of these several techniques of subthreshold laser treatment in various causes
of macular edema and macular pathologies.
Author’s affiliation
Defne
Kalayc MD
Prof of
Ophthalmology
Health
Sciences University, Ankara Numune Research and Training Hospital, Ankara,
Turkey.
dakalayci@hotmail.com
REFERENCES
1.
Early Treatment Diabetic Retinopathy Study Research Group: Photocoagulation
for diabetic macular edema: early treatment diabetic retinopathy study report
number 1. Arch Ophthalmol. 1985; 103: 1796–1806.
2.
Early Treatment Diabetic Retinopathy Study Research
Group: Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Ophthalmology, 1991 (98); 5 Suppl: 766-85.
3.
Hudson C, Flanagan JG, Turner
GS, et al. Influence of laser photocoagulation for clinically significant diabetic macular oedema
(DMO) on short-wavelength and conventional automated perimetry. Diabetologia.
1998; 41: 1283–1292.
4.
Schatz H, Madeira D, McDonald
HR, Johnson RN: Progressive enlargement of laser scars following grid laser photocoagulation
for diffuse diabetic macular edema. Arch Ophthalmol. 1991; 109: 1549–1551.
5.
Lewis H, Schachat AP, Haimann
MH, et al. Choroidal neovascularization after laser photocoagulation for
diabetic macular edema. Ophthalmology, 1990; 97: 503–510.
6.
Guyer DR, D’Amico DJ, Smith CW. Subretinal fibrosis after laser
photocoagulation for diabetic macular edema. Am J Ophthalmol. 1992 (113); 6: 652–656.
7.
Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, Edwards AR, Ferris FL 3rd, Friedman SM,Glassman AR, Miller KM, Scott IU, Stockdale CR, Sun JK. Diabetic Retinopathy Clinical Research Network. Randomized
Trial Evaluating Ranibizumab Plus Prompt or Deferred Laser or Triamcinolone
Plus Prompt Laser for Diabetic Macular Edema. Ophthalmology, 2010 (117); 6: 1064-1077.
8.
Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P,
Schlingemann RO, Sutter F, Simader C, Burian G, Gerstner O, Weichselberger A. Restore study group: The RESTORE study: ranibizumab monotherapy
or combined with laser versus laser monotherapy for diabetic macular edema.
Ophthalmology, 2011 (118); 4: 615-625.
9.
Cavalcante LL, Cavalcante ML, Murray
TG, Vigoda MM, Pińa Y, Decatur CL, Davis RP, Olmos LC, Schefler AC, Parrott MB,
Alliman KJ, Flynn HW, Moshfeghi AA. Intravitreal
injection analysis at the Bascom Palmer Eye Institute: evaluation of clinical
indications for the treatment and incidence rates of endophthalmitis. Clin Ophthalmol. 2010 (25); 4: 519-254.
10.
Brown DM, Nguyen QD, Marcus DM, Boyer
DS, Patel S, Feiner L, Schlottmann PG, Rundle AC, Zhang J, Rubio RG, Adamis AP,
Ehrlich JS, Hopkins JJ. RIDE and RISE Research Group: Long-term outcomes
of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology, 2013 (120); 10: 2013-22.
11. Wells JA, Glassman AR, Ayala AR, Jampol LM, Aiello LP, Antoszyk AN, Arnold-Bush B, Baker CW, Bressler NM, Browning DJ, Elman MJ, Ferris FL, Friedman SM, Melia M, Pieramici DJ, Sun JK, Beck RWN. Aflibercept,
bevacizumab, or ranibizumab for diabetic macular
edema. Diabetic Retinopathy Clinical Research Network. Engl J Med. 2015 (372); 13: 1193-1203.
12.
Wilson AS, Hobbs BG, Shen WY, Speed TP, Schmidt U, Begley CG, Rakoczy PE. Argon laser photocoagulation-induced modification of gene
expression in the retina. Invest Ophthalmol Vis Sci. 2003 (44); 4: 1426-1434.
13.
Dorin G. Evolution of retinal laser therapy: minimum intensity
photocoagulation (MIP). Can the laser heal the retina without harming it? Semin Ophthalmol.
2004 (19); (1-2): 62-68.
14.
Paulus YM, Jain A, Gariano RF, Stanzel BV, Marmor M, Blumenkranz MS, Palanker D. Healing of retinal
photocoagulation lesions. Invest Ophthalmol Vis Sci. 2008 (49); 12: 5540–5545.
15.
Roider J, Michaud NA, Flotte
TJ, Birngruber R. Response of the retinal pigment epithelium to selective
photocoagulation. Arch Ophthalmol. 1992 (110); 12: 1786–1792.
16. Luttrull JK, Dorin G. Subthreshold diode micropulse laser photocoagulation (SDM)
as invisible retinal phototherapy for diabetic macular edema: a review. Curr Diabetes Rev.
2012 (8); 4: 274-284.
17. Pankratov MM. Pulsed delivery of laser energy in experimental
thermal retinal photocoagulation. Proc
Soc Photo-Optical
Instrum Eng. 1990; 1202: 205–213.
18. Figueira J, Khan J, Nunes S, Sivaprasad S, Rosa A, de Abreu JF, Cunha-Vaz JG, Chong NV. Prospective randomised controlled trial comparing
sub-threshold micropulse diode laser photocoagulation and conventional green
laser for clinically significant diabetic macular oedema. Br J Ophthalmol. 2009 (93); 10: 1341-1344.
19. Lavinsky D, Cardillo JA, Melo LA Jr, Dare A, Farah ME, Belfort R Jr. Randomized clinical trial evaluating mETDRS versus normal
or high-density micropulse photocoagulation for diabetic macular edema. Invest Ophthalmol Vis Sci. 2011
(52); 7: 4314-4323.
20. Vujosevic S, Bottega E, Casciano M, Pilotto E, Convento E, Midena E. Microperimetry and fundus autofluorescence in diabetic
macular edema: subthreshold micropulse diode laser versus modified early
treatment diabetic retinopathy study laser photocoagulation. Retina, 2010
(30); 6: 908-916.
21. Venkatesh P, Ramanjulu R, Azad R, Vohra R, Garg S. Subthreshold micropulse diode laser and
double frequency neodymium: YAG laser in treatment of diabetic macular edema: a
prospective, randomized study using multifocal electroretinography. Photomed Laser Surg. 2011
(29); 11: 727-33.
22. Kalaycı D, Gültekin B, Baysan A,
Serdar K. Yellow Subthreshold
Micropulse Laser Treatment in Diabetic Macular Edema-Preliminary Results: J of
Retina-Vitreous, 2017 (26); 3: 228-231.
23. Chen G, Tzekov R, Li W, Jiang F, Mao S,
Tong Y. Subthreshold micropulse diode laser versus conventional laser
photocoagulation for diabetıc macular edema: A Meta-Analysis of Randomized
Controlled Trials. Retina, 2016 (36); 11: 2059-2065.
24. Lavinsky D, Wang J, Huie P, Dalal R, Lee SJ, Lee DY,
Palanker D. Nondamaging retinal laser therapy:rationale and applications to
the macula. Invest Ophthalmol Vis Sci. 2016; 57: 2488–2500.
25. Lavinsky D, Sramek C, Wang J, Huie P, Dalal R, Mandel Y, Palanker D. Subvisible Retinal Laser
Therapy: Titration Algorithm and Tissue Response. Retina, 2014 (34); 1 : 87-97.
26. Sramek C, Mackanos M, Spitler R, Leung LS, Nomoto H, Contag CH, Palanker D. Non-damaging retinal
phototherapy: dynamic range of heat shock protein expression. Invest Ophthalmol
Vis Sci. 2011 (52); 3: 1780-1787.
27. Pelosini L, Hamilton R, Mohamed M, Hamilton AM,Marshall J. Retina rejuvenation therapy
for diabetic macular edema: a pilot study. Retina, 2013 (33); 3: 548-58.
28. Schuele G, Rumohr M, Huettmann G, Brinkmann R. RPE damage thresholds and mechanisms for laser exposure in the
microsecond-to-millisecond time regimen. Invest
Ophthalmol Vis Sci. 2005(46); 2: 714-719.
29. Park YG, Kim JR, Kang S, Seifert E, Theisen-Kunde D, Brinkmann R, Roh YJ. Safety and efficacy of selective retina therapy (SRT) for
the treatment of diabetic macular edema in Korean patients. Graefes Arch Clin Exp Ophthalmol. 2016
(245); 9: 1703-1713.